Suture Material
DemeTECH Polyester Nonabsorbable Suture is a nonabsorbable, sterile, surgical suture composed of Poly (ethylene terephthalate). It is prepared from fibers of high-molecular weight, long-chain linear polyester having recurrent aromatic rings as an integral component. The product is available in several suture lengths and attached needle types. DemeTECH Polyester Nonabsorbable Suture meets all requirements established by the United States Pharmacopeia (USP) for Nonabsorbable Surgical Sutures.
Polyester (Braided) (PB)
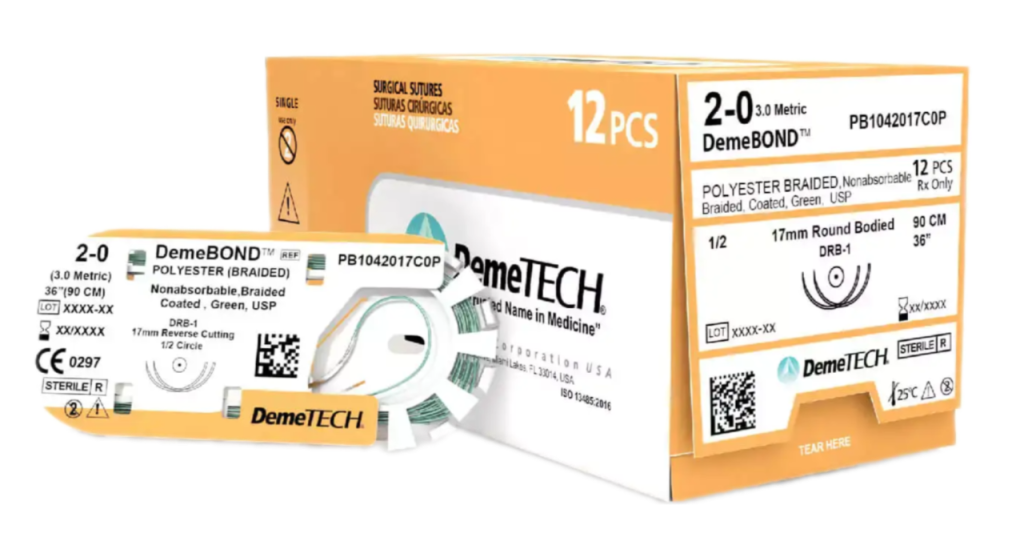
DemeBOND ™ Polyester (Braided) (PB)
INTENDED USE
The DemeTECH Nonabsorbable Polyester Suture with and without needles is indicated for use in general soft tissue approximation and/or ligation, including use in ophthalmic procedures. Product is intended for long term use over 30 days.
ACTIONS
The DemeTECH Polyester Nonabsorbable Suture elicits a minimal acute inflammatory reaction in tissues, followed by gradual encapsulation of the suture by fibrous connective tissue. DemeTECH Polyester Nonabsorbable Suture is not absorbed, nor is any significant change in tensile strength retention known to occur in vivo.
Characteristics
DemeTech’s Polyester suture is a nonabsorbable, sterile, surgical suture composed of Poly (ethylene terephthalate). DemeTech uniformly coats its Polyester sutures, providing for an improvement in the physical properties of the suture. High quality materials, combined with precise braiding mechanisms, nearly eliminate the occurrence of post operative suture fragments in the tissue. – Coated Polyester allows for an easier passage of the braided strands through the tissue allowing for smoother usage and less irritation. – It provides for minimal breakage, and this eliminates the need to remove irritating suture fragments postoperatively. – Provides excellent pliability (malleable). – Excellent handling qualities. – Smooth tie-down properties. – Dyed for good visibility in the surgical arena.
WARNINGS:
• Do not resterilize. Discard open, unused sutures. • As with any foreign body, prolonged contact of this or any other suture with salt solutions, such as those found in the urinary of biliary tracts, may result in calculus formation. • Users should be familiar with surgical procedures and techniques involving nonabsorbable sutures before employing the DemeTECH Polyester Nonabsorbable Suture for wound closure, as risk of wound dehiscence may vary with the site of application and the suture material used. • Acceptable surgical practice must be followed with respect to drainage and closure of infected or contaminated wounds. • Do not reuse. The reuse of single-use devices can cause cross contamination and affect the device safety, performance and effectiveness, exposing patients and staff to unnecessary risk. The design and material used are not compatible with conventional cleaning and sterilization procedures.
PRECAUTIONS
In handling this or any other suture material, care should be taken to avoid damage from handling. Avoid crushing or crimping damage due to application of surgical instruments such as forceps or needle holders. Adequate knot security requires the accepted surgical technique of flat, square ties, with additional throws as warranted by surgical circumstance and the experience of the surgeon. The use of additional throws may be particularly appropriate when knotting monofilaments.
ADVERSE REACTIONS
Adverse effects associated with the use of this device include: wound dehiscence, calculi formation in urinary and biliary tracts when prolonged contact with salt solutions such as urine and bile occurs, enhanced bacterial infectivity, minimal acute inflammatory tissue reaction, pain, edema, and erythema at the wound site.
DELIVERY FORM:
The DemeTECH Polyester Nonabsorbable Suture is available in sizes 8-0 through 2. The suture is supplied sterile in pre-cut lengths with affixed needles of various straight and curved needle types and supplied in cartons of one dozen.


